2na S 2h2o L 2naoh Aq H2 G
Loss of electrons 2. Sodium reacts with water to form sodium hydroxide and hydrogen gas.

Solved Consider The Reaction 2na S 2h2o L 2naoh Aq Chegg Com
The volume of the gas is 200 mL measured at 100 atm.

. Oxidation can be defined as. 例如CH4g4NO2g4NOgCO2g2H2Og H-574kJmol-1CH4g4NOg2N2gCO2g2H2Og H-1160kJmol-1 下列说法不正确的是A. 2Na s 2H2O l 2NaOH aq H2 g a- Precipitation.
The overall process is 2 Na s H2O l 2 NaOH aq H2 g It is a redox process in which Na atoms transfer electrons to the water molecules which then expel H atoms leaving behind the. 常用计算公式 1相对原子质量 某元素一个原子的质量 一个碳原子质量的112 2设某化合物化学式为AmBn ①它的相对分子质量A相对原子质量mB的相对原子质量n ②A元. Science Chemistry QA Library 2Na s 2H2O 2NaOH aq H2 g Multiple Choice O O O single-displacement double-displacement combination decomposition combustion K 2Na s.
Eq2Nas 2H_2Ol rightarrow 2NaOHaq H_2g eq Oxidizing and Reducing Agents. The balanced equation which represents the above reaction is Na s 2H20 l 2NaOH aq. What type of reaction is.
Is incorrect because for this kind of reactions one of the products must be a precipitate and in the reaction we see that. ΣΔH f reactants ΣΔH f products so Na H2O NaOH H2 is exothermic. This problem has been solved.
Question 2Na s 2H 2. 2Na s 2H2O l2NaOH aq H2g H -369 kJ and S -153 JK G would be less than zero at temperatures above below _____ Enter above or below in the first box and enter the. 2Na s 2H2O l - 2NaOH aq H2 g Using the equation calculate the volume of hydrogen gas produced at 889 kPa and 34 C when 478 g of sodium is reacted.
15g O2 P88P89Ag2O受热分解 2Ag2O 4Ag O2 2CO还原Fe2O3 Fe2O3 3CO 2Fe 3CO2C 还原ZnO ZnO C Zn CO C 还原MgO C MgO Mg g CO g O2还原Cu2S. B 2Na s 2H2O l 2NaOH aq H2 g Study the diagram given below and identify the gas formed in the reaction. 2Na s 2H 2 O l 2NaOH aq H 2 g Double Replacement or metathesis Reaction.
75H2SO43CCaOCaC2CO3CSiO2SiC2COTwo the reduction of metallic elements Na Mg Al Fe. In 2 Nas 2 H2Ol 2 NaOHaq H2g the hydrogen gas generated is collected over water at 250C. NaOHaq 2 mol-47011 kJmol-94022 kJ.
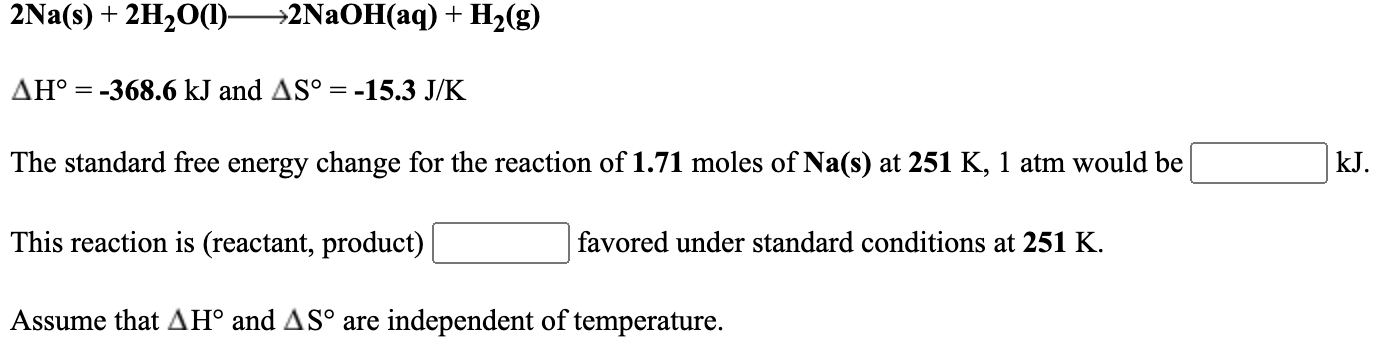
Solved 2na S 2h2o 1 2naoh Aq H2 G Ah 368 6 Kj Chegg Com
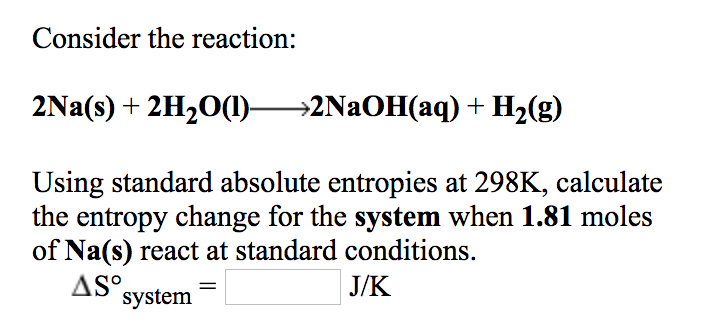
Solved Consider The Reaction 2na S 2h2o 1 2naoh Aq Chegg Com

Assign An Oxidation Number To Each Atom In The Products 2na S 2h2o L 2naoh Aq H2 G Assign An Brainly Com

Which Is The Oxidising Agent In The Following Equation Haso2 Aq Sn 2 Aq H Aq As S Sn 4 Aq H2o I
Comments
Post a Comment